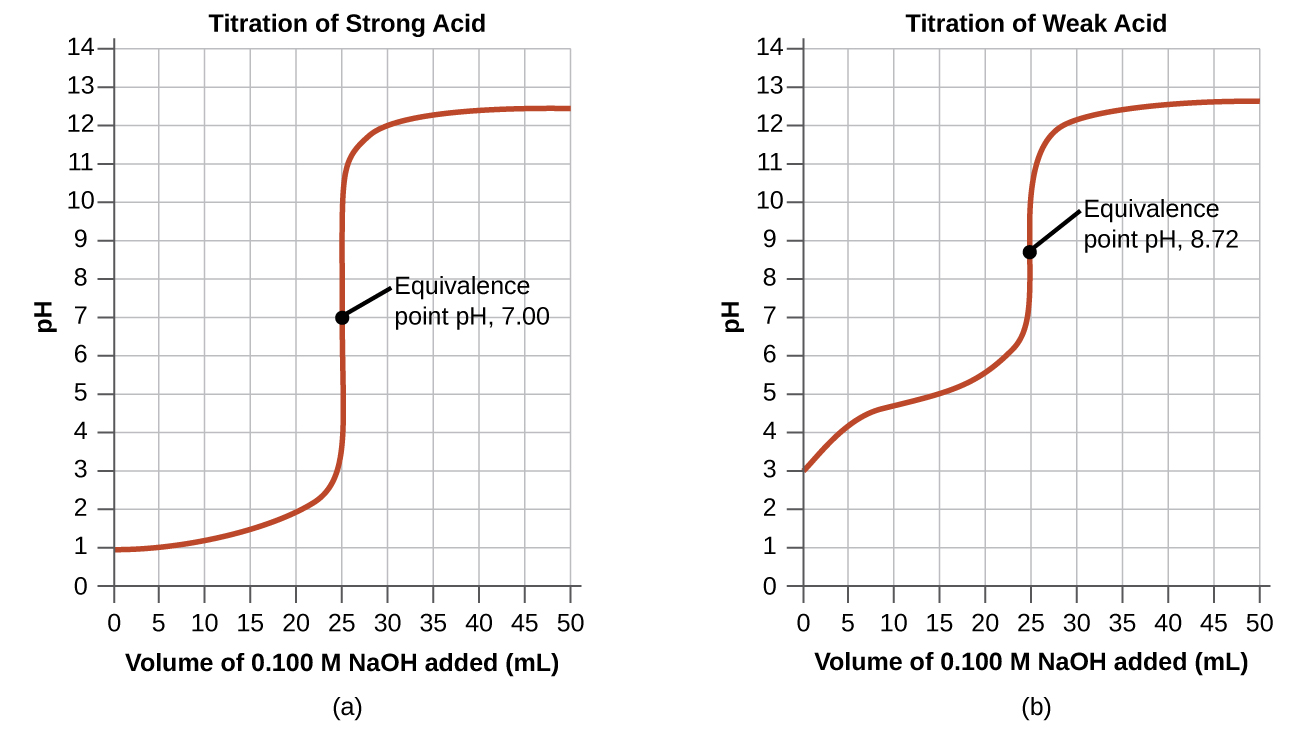
Titration Curve Equivalence Point And Endpoint . the main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical. Endpoint is represented as a sharp peak or dip on the titration curve. A point of equivalence in a. during the process, two important stages known as endpoint and equivalence point are reached. what is the difference between the end point and the equivalence point? The term equivalence point means that the solutions have been mixed. Equivalence point is represented as the. The end point is simply when a colour change occurs as the solution in a titration gets. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. The term neutral point is best avoided.
from exokpafwp.blob.core.windows.net
Endpoint is represented as a sharp peak or dip on the titration curve. the main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical. during the process, two important stages known as endpoint and equivalence point are reached. The term equivalence point means that the solutions have been mixed. The end point is simply when a colour change occurs as the solution in a titration gets. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. what is the difference between the end point and the equivalence point? A point of equivalence in a. The term neutral point is best avoided. Equivalence point is represented as the.
Titration Endpoint Calculator at Kevin Dowell blog
Titration Curve Equivalence Point And Endpoint to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. The term equivalence point means that the solutions have been mixed. The end point is simply when a colour change occurs as the solution in a titration gets. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. Endpoint is represented as a sharp peak or dip on the titration curve. Equivalence point is represented as the. The term neutral point is best avoided. during the process, two important stages known as endpoint and equivalence point are reached. A point of equivalence in a. the main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical. what is the difference between the end point and the equivalence point?
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Equivalence Point And Endpoint The term equivalence point means that the solutions have been mixed. what is the difference between the end point and the equivalence point? to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. during the process, two important stages known as endpoint and equivalence point are reached. Equivalence. Titration Curve Equivalence Point And Endpoint.
From exokpafwp.blob.core.windows.net
Titration Endpoint Calculator at Kevin Dowell blog Titration Curve Equivalence Point And Endpoint The term neutral point is best avoided. during the process, two important stages known as endpoint and equivalence point are reached. what is the difference between the end point and the equivalence point? The end point is simply when a colour change occurs as the solution in a titration gets. The term equivalence point means that the solutions. Titration Curve Equivalence Point And Endpoint.
From chemistrytalk.org
Titration Curves & Equivalence Point Calculations ChemTalk Titration Curve Equivalence Point And Endpoint what is the difference between the end point and the equivalence point? The end point is simply when a colour change occurs as the solution in a titration gets. The term neutral point is best avoided. A point of equivalence in a. during the process, two important stages known as endpoint and equivalence point are reached. the. Titration Curve Equivalence Point And Endpoint.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Curve Equivalence Point And Endpoint The term neutral point is best avoided. during the process, two important stages known as endpoint and equivalence point are reached. The end point is simply when a colour change occurs as the solution in a titration gets. A point of equivalence in a. The term equivalence point means that the solutions have been mixed. the main difference. Titration Curve Equivalence Point And Endpoint.
From exoliotyy.blob.core.windows.net
Equivalence Point Titration Example at Daniel Hoggard blog Titration Curve Equivalence Point And Endpoint to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. Equivalence point is represented as the. the main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical. The term neutral point is best avoided. what is the difference between the. Titration Curve Equivalence Point And Endpoint.
From schoolbag.info
Titration and Buffers Acids and Bases Titration Curve Equivalence Point And Endpoint The term equivalence point means that the solutions have been mixed. during the process, two important stages known as endpoint and equivalence point are reached. A point of equivalence in a. The term neutral point is best avoided. the main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical. . Titration Curve Equivalence Point And Endpoint.
From ar.inspiredpencil.com
Equivalence Point And Endpoint Titration Curve Equivalence Point And Endpoint what is the difference between the end point and the equivalence point? The term equivalence point means that the solutions have been mixed. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. The end point is simply when a colour change occurs as the solution in a titration. Titration Curve Equivalence Point And Endpoint.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Equivalence Point And Endpoint to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. what is the difference between the end point and the equivalence point? Endpoint is represented as a sharp peak or dip on the titration curve. The end point is simply when a colour change occurs as the solution in. Titration Curve Equivalence Point And Endpoint.
From www.vrogue.co
How To Find Equivalence Point From Titration Data vrogue.co Titration Curve Equivalence Point And Endpoint the main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical. The term equivalence point means that the solutions have been mixed. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. Equivalence point is represented as the. A point of. Titration Curve Equivalence Point And Endpoint.
From philschatz.com
AcidBase Titrations · Chemistry Titration Curve Equivalence Point And Endpoint A point of equivalence in a. during the process, two important stages known as endpoint and equivalence point are reached. what is the difference between the end point and the equivalence point? Equivalence point is represented as the. The term equivalence point means that the solutions have been mixed. Endpoint is represented as a sharp peak or dip. Titration Curve Equivalence Point And Endpoint.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration Curve Equivalence Point And Endpoint A point of equivalence in a. Endpoint is represented as a sharp peak or dip on the titration curve. Equivalence point is represented as the. The term equivalence point means that the solutions have been mixed. the main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical. what is the. Titration Curve Equivalence Point And Endpoint.
From mungfali.com
Endpoint Titration Curve Titration Curve Equivalence Point And Endpoint during the process, two important stages known as endpoint and equivalence point are reached. Equivalence point is represented as the. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. the main difference between equivalence point and endpoint is that equivalence point is the actual point where the. Titration Curve Equivalence Point And Endpoint.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Equivalence Point And Endpoint The term equivalence point means that the solutions have been mixed. A point of equivalence in a. during the process, two important stages known as endpoint and equivalence point are reached. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. what is the difference between the end. Titration Curve Equivalence Point And Endpoint.
From www.youtube.com
How to Find the Equivalence Point on a Titration Graph In Excel YouTube Titration Curve Equivalence Point And Endpoint Equivalence point is represented as the. during the process, two important stages known as endpoint and equivalence point are reached. what is the difference between the end point and the equivalence point? The term equivalence point means that the solutions have been mixed. The end point is simply when a colour change occurs as the solution in a. Titration Curve Equivalence Point And Endpoint.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Equivalence Point And Endpoint during the process, two important stages known as endpoint and equivalence point are reached. what is the difference between the end point and the equivalence point? The term equivalence point means that the solutions have been mixed. Equivalence point is represented as the. A point of equivalence in a. The end point is simply when a colour change. Titration Curve Equivalence Point And Endpoint.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Equivalence Point And Endpoint The term equivalence point means that the solutions have been mixed. the main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical. A point of equivalence in a. The term neutral point is best avoided. Equivalence point is represented as the. during the process, two important stages known as endpoint. Titration Curve Equivalence Point And Endpoint.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Chemistry JoVe Titration Curve Equivalence Point And Endpoint during the process, two important stages known as endpoint and equivalence point are reached. A point of equivalence in a. The term equivalence point means that the solutions have been mixed. Endpoint is represented as a sharp peak or dip on the titration curve. what is the difference between the end point and the equivalence point? The term. Titration Curve Equivalence Point And Endpoint.
From romeo-blogmeza.blogspot.com
Explain Equivalence Point and End Point Titration Curve Equivalence Point And Endpoint the main difference between equivalence point and endpoint is that equivalence point is the actual point where the chemical. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. Equivalence point is represented as the. The term equivalence point means that the solutions have been mixed. during the. Titration Curve Equivalence Point And Endpoint.